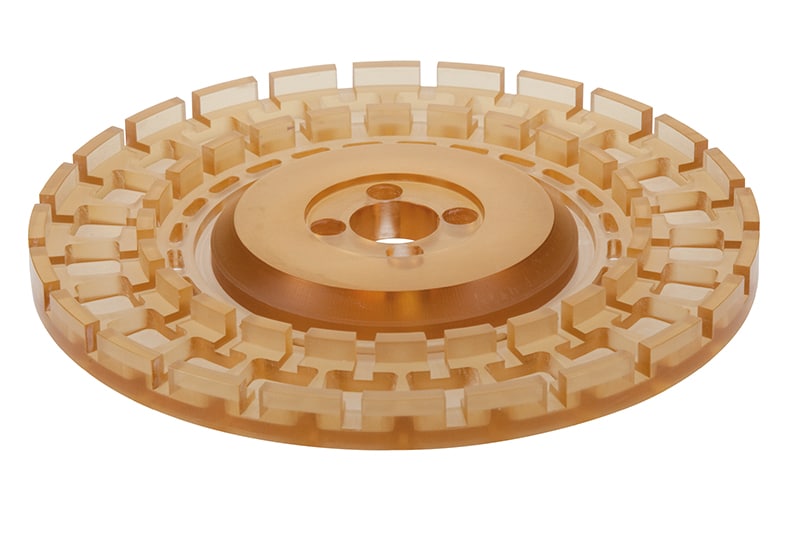
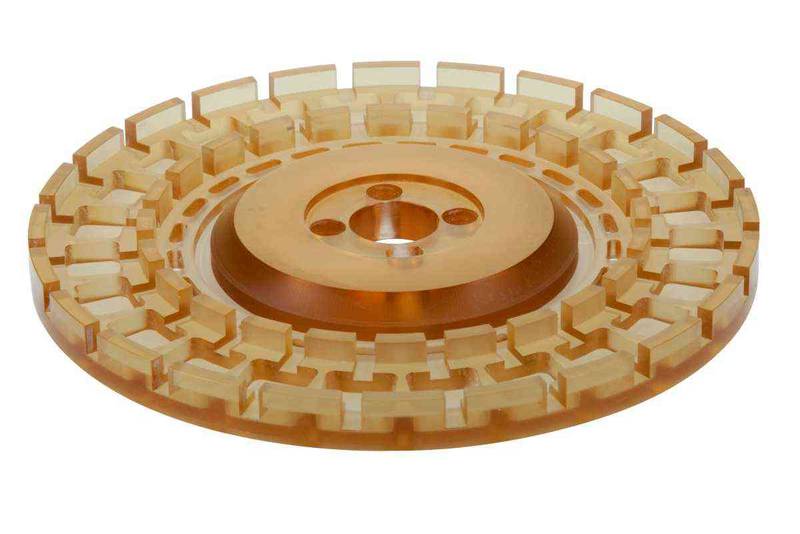
Plastic parts for medical technology
In medical technology, the highest demands are placed on the processing and material for the use of plastic parts. High surface finishes, very tight tolerances, absolute freedom from chips and burrs, certificates and traceability are the daily business here, which plastic parts must meet in order to fulfill the requirements of use in medical manufacturing. To ensure that the plastic parts produced meet these requirements, special experience and expertise in manufacturing are needed. Through our long-standing partnerships with many well-known companies in this industry, we have accumulated a wealth of experience and reliable know-how with which to meet the ever-increasing demands of this sector.
In medical & medical device technology, mainly plastics and materials are used that have the following properties:
- physiological safety (FG – FoodGrade, FDA or EU conformity according to EU 10/2011, 1935/2004 and 2023/2006 GMP)
- biocompatible (MG – MedicalGrade, biocompatible according to ISO 10993 or USP Class VI)
- good chemical resistance – resistant to many cleaning agents
- Hydrolysis & Sterilization Resistance
- High mechanical strength, stiffness and dimensional stability
- low moisture absorption
- Temperature resistance over a wide temperature range
- Laser marking possible – Traceability